Abstract
CD37 is a tetraspanin molecule expressed on mature B-cells, but absent on normal stem cells and plasma cells. Due to this cellular distribution, which is highly similar to CD20, CD37 has gained attention as a target for B-cell lymphoma, especially for rituximab-resistant and relapsed patients.
The CD37-targeting antibody-based products that are currently being evaluated in the clinic are generally poor inducers of complement-dependent cytotoxicity (CDC) - a rapid and powerful Fc-mediated effector function. DuoHexaBody-CD37 is a bispecific IgG1 antibody targeting two non-overlapping epitopes in the large extracellular loop of CD37, with a hexamerization-enhancing mutation (E430G) in the IgG Fc domain. The E430G mutation increases the intrinsic capacity of IgG1 molecules to form hexameric antibody clusters upon binding to membrane-bound antigens, thereby facilitating binding of the hexavalent complement component C1q and subsequent CDC activity (Diebolder et al., Science 2014; de Jong et al., PLoS Biology 2016). The combination of dual epitope targeting and enhanced hexamerization of DuoHexaBody-CD37 upon binding to the cell surface significantly increases complement activation and CDC efficacy in CD37-positive B-cells.
To assess its clinical relevance and potential, we tested the capacity of DuoHexaBody-CD37 to induce ex vivo CDC activity using a broad range of B-cell lymphoma and leukemia patient samples (Diffuse Large B-Cell Lymphoma, Follicular Lymphoma, Mantle Cell Lymphoma, Marginal Zone Lymphoma, Chronic Lymphocytic Leukemia, n=40) derived from both newly diagnosed (ND) (n=23) and relapsed and/or refractory (RR) patients (n=17), most of whom had received prior treatment with rituximab. The CDC activity of DuoHexaBody-CD37 was compared to the CDC activity of the CD20 antibodies rituximab, ofatumumab and obinutuzumab. Our results revealed that, independent of the B-cell malignancy subtype, DuoHexaBody-CD37 induced potent CDC activity with a median lysis of 82% (n=40, both RR and ND) at 10 µg/ml, and significantly outperformed all three tested CD20 antibodies (18%, 24% and 51% median lysis for rituximab, obinutuzumab and ofatumumab, respectively). Furthermore, CDC induced by DuoHexaBody-CD37 was shown to be less sensitive to the expression of complement regulatory proteins (CD46, CD55, CD59), compared to the tested CD20 antibodies, although the few patient samples that were resistant to DuoHexaBody-CD37 in the ex vivo CDC assays expressed significantly higher CD59 levels than the responsive samples.
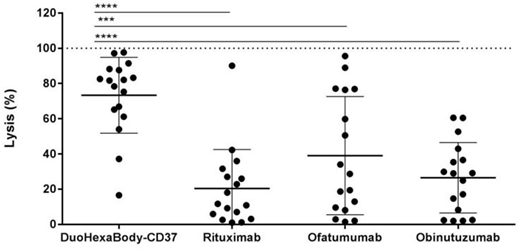
Importantly, while CDC induced by the tested CD20 antibodies was lower in samples derived from RR patients than from ND patients, DuoHexaBody-CD37 was equally effective in inducing CDC in samples derived from ND and RR patients that received prior treatment with rituximab. In a direct comparison to the strong CDC inducer ofatumumab, DuoHexaBody-CD37 induced significantly more potent CDC in samples from 17 RR CLL and B-cell lymphoma patients with a median lysis of 82% for DuoHexaBody-CD37 versus 29% for ofatumumab (p<0.001) (Fig. 1).
In conclusion, our results indicate great therapeutic potential for DuoHexaBody-CD37 in B cell malignancies, based on its strong capacity to induce CDC, even in ex vivo samples from patients that had relapsed from or were refractory to rituximab-containing treatments.
Figure1 Complement-dependent cytotoxicity induced by DuoHexaBody-CD37, rituximab, ofatumumab or obinutuzumab at 10 µg/mL in frozen CLL or lymphoma B-cells of relapsed and/ or refractory patients that received prior treatment with rituximab (n=17). Statistical analysis was performed using a non-parametric t-test (Wilcoxon). ***p < 0.001 and ****p < 0.0001. Data is shown as mean ± SD.
Van Der Horst:Genmab: Research Funding. Oostindie:Genmab: Employment, Equity Ownership. Overdijk:Genmab: Employment, Equity Ownership. Chamuleau:Gilead: Research Funding; Genmab: Research Funding; celgene: Research Funding; BMS: Research Funding. Breij:Genmab: Employment, Equity Ownership. Mutis:Celgene: Research Funding; Novartis: Research Funding; Gilead: Research Funding; OnkImmune: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genmab: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal